Alcohol and GABA: Why You Stagger, Slur, and Forget Things When Drunk
By Kenneth Anderson, MA
Alcohol and the Cerebellum
Some drugs like opioids, benzodiazepines, and amphetamines have been called chemical scalpels because each affects only a single neurotransmitter system. Alcohol, on the other hand, is a dirty drug which affects large numbers of neurotransmitter systems, including GABA (gamma-aminobutyric acid), glutamate, and dopamine. Hence, alcohol has been referred to as a chemical hand grenade. In this post, we will be looking at alcohol’s effects on the GABA system to see how it causes staggering (aka ataxic gait) and slurring of speech (dysarthrias), sedation, and memory loss. First, let’s take a look at the GABA receptors.
Although there is only one type of GABA, there are two types of GABA receptors: type A, which are known as GABA-A receptors, and type B, which are known as GABA-B receptors. The letters A and B are just arbitrary designations with no meaning attached to them. For our purposes, we are only interested in the GABA-A receptors.
Each GABA-A receptor is comprised of five subunits, arranged in the shape of a pentagon around a central pore, known as an ion channel. There are eight classes of subunits which can be used to construct a GABA-A receptor; these classes are named using the Greek letters alpha, beta, gamma, delta, epsilon, pi, rho, and theta. The alpha subunit is further divided into six subtypes: alpha(1), alpha(2), alpha(3), alpha(4), alpha(5), and alpha(6). The beta, gamma, and rho subtypes are each divided into three subtypes: beta(1), beta(2), and beta(3); gamma(1), gamma(2), and gamma(3); and rho(1), rho(2), and rho(3). The rest have no subtypes.
The typical GABA-A receptor is comprised of two alpha subunits, two beta subunits, and one additional subunit which can be a gamma, delta, epsilon, pi, or theta subunit. (The rho subunits are special and won’t be discussed here as they are not involved in the effects of alcohol.) GABA binds in a pocket between the alpha and beta subunits. For our purposes, we will limit ourselves to looking at GABA-A receptors comprised of alpha, beta, and delta subunits, which are the GABA-A receptors affected by alcohol, and GABA-A receptors comprised of alpha, beta, and gamma subunits, which are the GABA-A receptors affected by benzodiazepines.
Each GABA-A receptor subunit is a protein. A protein is a chain of chemically bonded amino acids. Within classes, subunits show a 60-70% similarity in the sequence of amino acids making up the protein chain. In other words, the amino acid sequence of subunit alpha(1) is about 60-70% similar to that of subunit alpha(2), and so on. The amino acid sequences between classes of subunits are only about 30-40% similar. In other words, the amino acid sequence of subunit alpha(1) is about 30-40% similar to that of beta(1) or beta(2), and so forth.
In order for the ion channel of the GABA-A receptor to open, it is necessary that two GABA molecules bind to the receptor. The GABA binding sites are located where the alpha subunit joins the beta subunit. Both of these binding sites must be occupied by a GABA molecule in order for the receptor to open and allow ions (i.e., electrical current) to flow between the inside and the outside of the cell membrane of the neuron. The receptor pore remains closed if no GABA molecule is bound or if only one GABA molecule is bound.
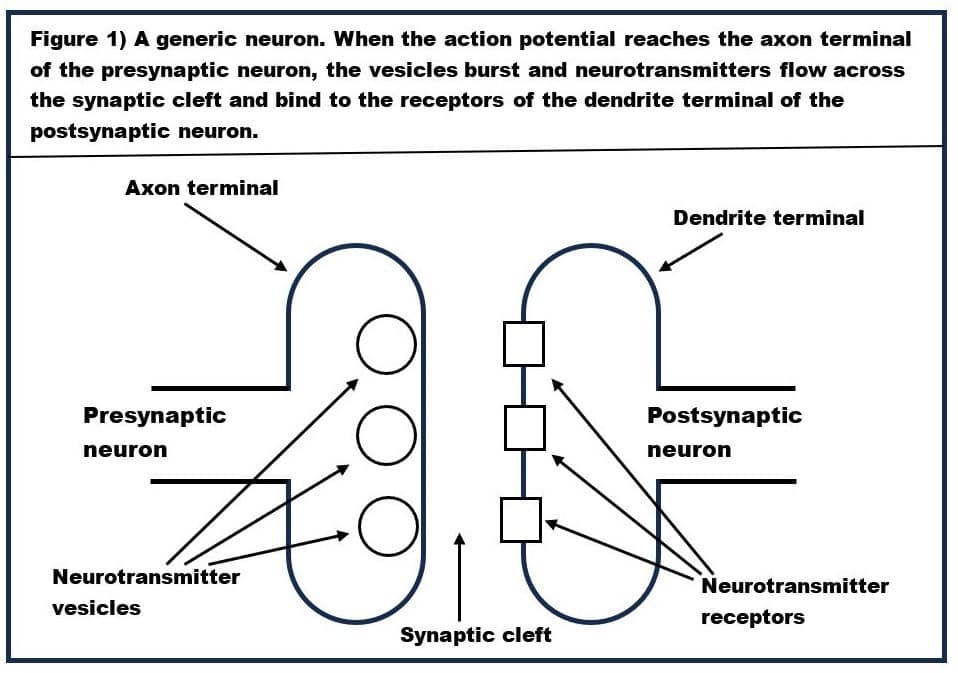
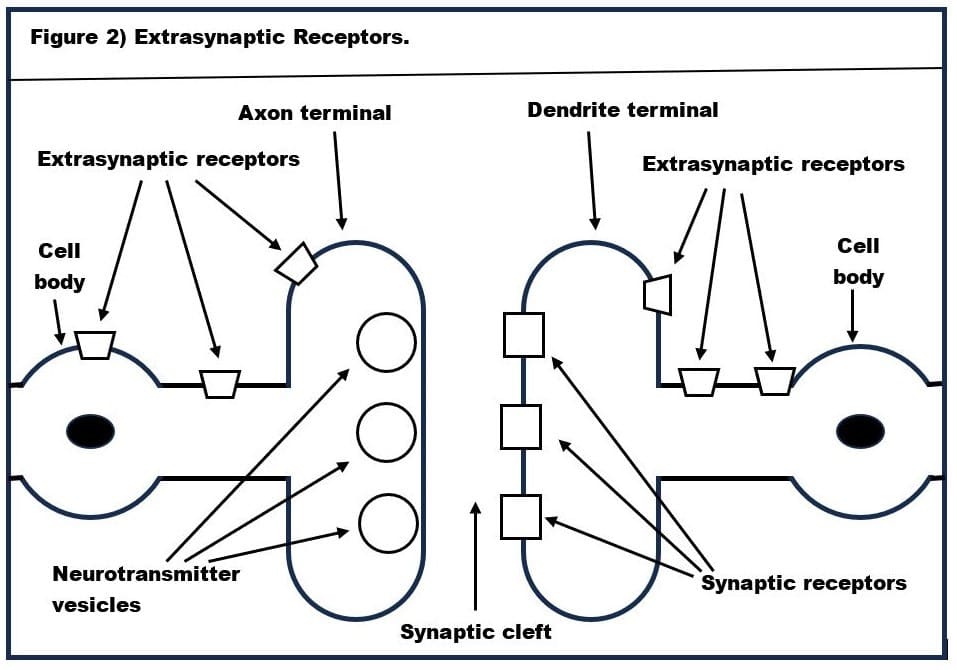
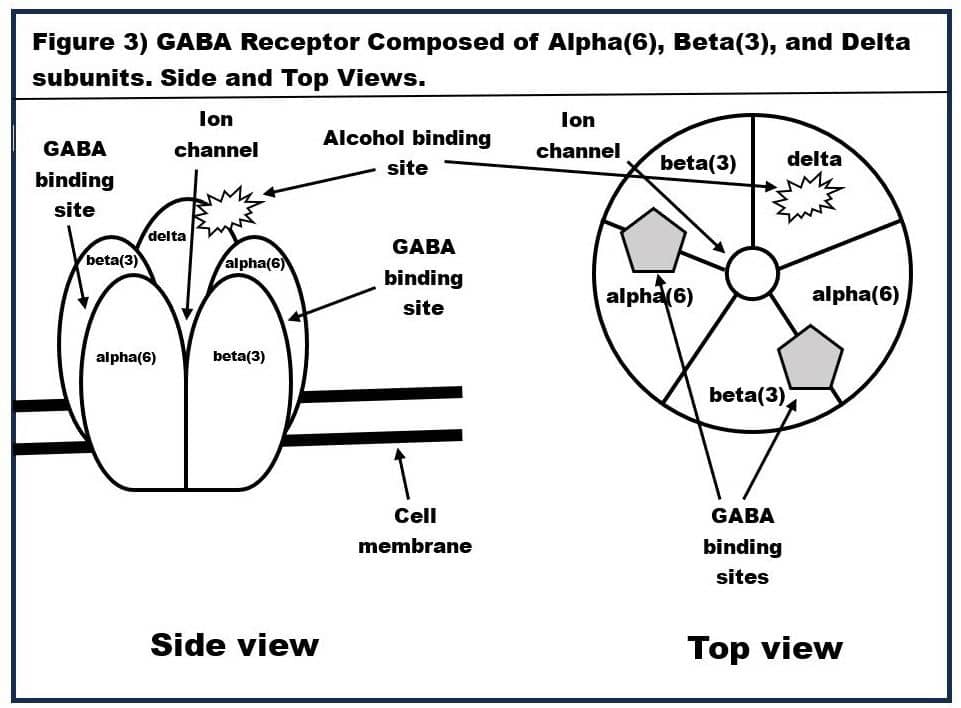
Moreover, alcohol (in normal doses) only binds to two very specific types of GABA-A receptors-‑these are alpha(6)beta(3)delta GABA-A receptors and alpha(4)beta-delta GABA-A receptors. The structure of the alpha(6)beta(3)delta GABA-A receptor is shown in Figure 3. Alpha(6)beta(3)delta GABA-A receptors are found only on the surface of the cerebellum’s granule cells. Since the cerebellum is the seat of fine motor control, this explains why alcohol causes staggering (aka ataxic gait) and slurring of speech (dysarthrias). Let’s look at how these alpha(6)beta(3)delta GABA-A receptors work.
Although it is well established that alcohol increases strength (i.e., amplitude) of the background firing of cerebellar granule cells, Valenzuela and Jotty (2015) present an alternative to the hypothesis that this is due to the direct effect of alcohol on the extrasynaptic receptors. Valenzuela and Jotty hypothesize that alcohol increases the firing rate of the Golgi cells in the cerebellum, and that GABA released by these Golgi cells binds to the extrasynaptic receptors on the granule cells and that this is what increases the amplitude of their background firing.
Alcohol and the Hippocampus, Thalamus, Striatum, and Neocortex
The alpha(4)beta-delta GABA-A receptor comes in two varieties: one variety is comprised of two alpha(4) subunits, two beta(2) subunits, and a delta subunit. The other variety is comprised of two alpha(4) subunits, two beta(3) subunits, and a delta subunit. There is not a great deal of difference between these two varieties, so, for the sake of simplicity we shall simply refer to them as alpha(4)beta-delta GABA-A receptors.
The alpha(4)beta-delta GABA-A receptors are extrasynaptic receptors found on the neurons of the hippocampal formation, the thalamus, the striatum, the neocortex, and a few other places. The hippocampal formation is the seat of memory; it is comprised of the hippocampus proper, the dentate gyrus, and a few other structures. The granule neurons of the dentate gyrus are covered in extrasynaptic alpha(4)beta-delta GABA-A receptors; when alcohol binds to these receptors and slows the background firing rate of these neurons it can interfere with memory formation.
The thalamus is a structure about the size and shape of a hen’s egg which is found deep in the brain. There is one thalamus in each hemisphere. The thalamus is the seat of arousal and pain regulation, among other things. Extrasynaptic alpha(4)beta-delta GABA-A receptors are found on the relay neurons of the thalamus. When alcohol binds to the extrasynaptic alpha(4)beta-delta GABA-A receptors of the thalamus it gives rise to the hypnotic, sedative, and pain-killing effects of alcohol.
Alcohol also binds to extrasynaptic alpha(4)beta-delta GABA-A receptors on the spiny neurons of the striatum, a deep-brain structure located near the thalamus. These neurons are involved in motor control, habit formation, and motivated behavior.
The human cerebrum consists of two parts: the gray matter on the outside, and the white matter on the inside. The gray matter on the outside of the cerebrum is known as the neocortex (“neo-” is a Latin prefix meaning “new” and “cortex” means “covering;” the neocortex covers the entire cerebrum). The neocortex is the seat of reason, among other things. When alcohol binds to the extrasynaptic alpha(4)beta-delta GABA-A receptors of the pyramidal cells in the neocortex it affects reasoning, and this can lead to poor decision making and disinhibition.
Benzodiazepines
Interestingly, the GABA-A receptors which bind alcohol are incapable of binding benzodiazepines and are known as benzodiazepine-insensitive receptors. Likewise, GABA-A receptors which bind benzodiazepines are incapable of binding alcohol at normal concentrations. GABA-A receptors which bind benzodiazepines are comprised of two alpha subunits, two beta subunits, and a gamma subunit. Those which bind to alcohol have a delta subunit instead of a gamma subunit.
The binding site for benzodiazepines is a pocket between the gamma subunit and the alpha subunit. GABA-A receptors which bind benzodiazepines can contain subunits alpha(1), alpha(2), alpha(3), alpha(5), gamma(2), gamma(3), and any of the betas. GABA-A receptors which contain alpha(4), alpha(6), or gamma(1) do not bind benzodiazepines or bind them very poorly. Unlike alcohol, benzodiazepines readily bind to either synaptic receptors or to extrasynaptic receptors, and hence, they can slow down either synaptic (phasic) firing or background (tonic) firing. The effect of alcohol on synaptic firing is much more limited. It is interesting that alcohol and benzodiazepines are cross-tolerant, since they bind to different types of GABA-A receptors.
Z-Drugs
The Z-drugs Ambien (zolpidem) and Sonata (zaleplon) bind only to the pocket between the alpha(1) subunit and the gamma subunit. This is why Ambien and Sonata are much more selective in their effects than benzodiazepines. The Z-drug Lunesta (eszopiclone), on the other hand, has a less selective binding profile than Ambien or Sonata.
Barbiturates
Barbiturates can also act to increase the binding of the GABA-A receptor, or, in larger doses they can cause the ion channel to open even when GABA is not bound to the receptor. (The technical term for this is allosteric agonism.) Since alcohol increases the rate at which the ion channel opens, whereas barbiturates increase the amount of time the ion channel is open, barbiturates and alcohol are synergistic in their effects. In other words, mixing alcohol with barbiturates is very dangerous and far more likely to kill you than mixing alcohol with benzodiazepines, although that can kill you too. Barbiturates are also synergistic with benzodiazepines, so that mixture is also quite deadly.
The Low-Affinity Alcohol Binding Site
There is another binding site for alcohol on GABA-A receptors located where the receptor passes through the cell membrane; however, this site can only be activated by near-lethal doses of alcohol, i.e., a BAC of 0.3 or higher. This is called the low-affinity alcohol binding site. However, since it is not involved in normal drunkenness, we shall not discuss it further.
Conclusion
Alcohol, benzodiazepines, Z-drugs, and barbiturates all have some similar effects in the brain because they all affect the GABA receptors. However, there are differences in the way these substances affect the brain because they each bind to different subtypes of GABA receptors. Alcohol causes more staggering, slurred speech, and incoordination than the others because of its fondness for binding to receptors in the cerebellum. Z-drugs primarily induce sleep because they affect only one subtype of GABA receptor. Benzodiazepines work best to relieve anxiety and cause relaxation because they have less effect on the cerebellum than alcohol. And barbiturates have largely been taken off the market because of their risk of overdose, especially when mixed with alcohol or benzodiazepines.
