THIQs Revisited
by Kenneth Anderson, M.A.
THIQ is an acronym which stands for tetrahydroisoquinoline, a chemical which is formed in the brain when a metabolite of alcohol combines with dopamine. The THIQ theory of “alcoholism” became very popular in the 1970s and 1980s, when it was erroneously believed that THIQs were formed in the brains of “alcoholics,” but not in the brains of normal drinkers. The theory became so popular that it was regularly taught to clients at Hazelden, both in a document riddled with historical errors and in a videotape which I recall seeing in the 1990s. THIQ theory is discussed in more detail below. Although THIQ theory was debunked in the 1990s when it was found that THIQs were formed equally well in the brains of both normal drinkers and “alcoholics,” recent research suggests that THIQs play a very important role in alcohol’s effects on the brain. But before we get there, let us first review some of the basics of neurotransmission.
Back to Basics: Neurotransmission
When the voltage difference between the outside and the inside of a neuron reaches a certain critical threshold, the neuron fires, sending an electrical pulse down the axon to the axon terminal (aka presynaptic terminal). When the electrical pulse reaches the axon terminal, it causes sacks in the axon terminal to burst open, releasing the neurotransmitters which they contain into the synaptic cleft (i.e., the gap between the axon terminal of the first neuron and the dendrite terminal of the second neuron). Some of these neurotransmitters cross the synaptic gap and bind to receptors on the terminal of dendrite of the second neuron (aka the postsynaptic terminal). This whole process only takes about 0.3 to 0.5 milliseconds. When the neurotransmitters bind to the receptors on the dendrite of the second neuron, this causes a change in the voltage difference between the outside and the inside of this neuron. If enough dendrites of the second neuron receive enough voltage changes to reach the critical threshold, the second neuron fires, affecting a third neuron, and so on, ad infinitum. This is prototypical neurotransmission, although as we shall see below, receptors can also be found on other parts of the neuron, including the axon terminal itself.
The Neurotransmission of Drugs
Drugs such as morphine, benzodiazepines, alcohol, naltrexone, etc. have their effects by binding to neurotransmitter receptors. Morphine is very simple in its effect, since it simply binds to the same place on the brain’s mu-opioid receptors as does beta-endorphin, the brain’s naturally occurring opioid. This is known as orthosteric binding, Since morphine binds to the same place on the mu-opioid receptor as beta-endorphin, and since it activates the receptor, morphine is known as an orthosteric agonist. Naltrexone also binds to the same place on the mu-opioid receptor as beta-endorphin; however, when naltrexone binds to the mu-opioid receptor, it blocks the activation of the receptor. Hence, naltrexone is known as an orthosteric antagonist.
GABA (gamma-aminobutyric acid) is the naturally occurring agonist of the GABA receptors. Benzodiazepines have their effect by binding to GABA receptors. However, benzodiazepines bind to the GABA receptor at a different spot than the one to which GABA binds. This is known as allosteric binding. Benzodiazepines do not activate the GABA receptors, rather, they alter the properties of the receptor, making it more receptive to GABA. Hence, benzodiazepines are known as allosteric modulators. GABA is the brain’s major inhibitory neurotransmitter system and is responsible for feelings of relaxation and calmness.
Although alcohol is one of the simplest psychoactive molecules in terms of its structure, it is one of the most complex in terms of its effects on the brain. Alcohol affects almost every receptor in the brain, including the GABA, glutamate, opioid, cannabinoid, and dopamine receptors. Alcohol is an allosteric modulator of the GABA receptors; it increases their activity, and this causes relaxation and calmness. Alcohol is also an allosteric modulator of the glutamate receptors. The glutamate system is the brain’s major excitatory neurotransmitter system. Alcohol decreases the activity of the glutamate system, and this also causes relaxation and calmness.
Alcohol also affects the brain’s opioid receptor system, and this, in turn, causes the release of dopamine into the brain’s nucleus accumbens. It is this release of dopamine into the brain’s nucleus accumbens which is responsible for alcohol’s rewarding and addictive effects. However, alcohol does not itself directly affect the opioid receptor system in the brain, rather, it is a metabolite of alcohol known as acetaldehyde which is responsible for the effects of alcohol on the opioid receptor system.
When the body breaks down alcohol, it does so with an enzyme which removes one hydrogen atom from the alcohol molecule, converting it into acetaldehyde, a highly toxic poison similar to formaldehyde. Very quickly, another enzyme steps in and converts the acetaldehyde into acetic acid, the essential component of vinegar. This acetic acid then enters the Krebs cycle and provides energy for cells while it is broken down into its component parts.
The primary locations where alcohol is metabolized are the stomach and the liver, however, all the cells in the body are capable of metabolizing alcohol, including the cells of the brain. Acetaldehyde has very different effects in the body than it does in the brain. Normally, acetaldehyde in the body is metabolized into acetic acid so quickly that it has little effect on the body. However, the drug antabuse, which is used as a deterrent to drinking alcohol, binds irreversibly to the enzyme which converts acetaldehyde to acetic acid and inactivates it, which causes acetaldehyde to build up in the body. The acetaldehyde causes flushing, throbbing in the head and neck, a throbbing headache, respiratory difficulty, nausea, copious vomiting, sweating, thirst, chest pain, palpitation, dyspnea, hyperventilation, fast heart rate, low blood pressure, fainting, marked uneasiness, weakness, vertigo, blurred vision, and confusion. In severe cases, this can lead to heart failure and death. Antabuse is effective at deterring alcohol consumption for up to two weeks after it is taken, while the liver is generating new enzymes to replace the deactivated enzymes.
Another instance where acetaldehyde builds up in the body is the so-called “Asian flush.” Some people produce a fairly inefficient variant of the enzyme which converts acetaldehyde to acetic acid, which allows acetaldehyde to accumulate in the body. This inefficient variant of the enzyme is usually found in Asians; about 30 to 50% of Chinese, Japanese, and Koreans show the Asian flush reaction.
Acetaldehyde rarely crosses the blood-brain barrier; it is usually metabolized before it has a chance to cross the barrier. The acetaldehyde which is found in the brain is usually a result of alcohol metabolism which takes place inside the brain itself. The acetaldehyde in the brain can combine with dopamine to form chemicals known as tetrahydroisoquinolines (THIQs). The history of THIQs is an interesting story.
The History of THIQs
THIQs have long been known to be precursors of morphine in the opium poppy. In the late 1960s, Virginia E. Davis, PhD (Nov 9, 1925 – Oct 30, 2012), a researcher at the Houston, Texas Veterans’ Administration, and Michael J. Walsh, PhD (Apr 18, 1942 – Jun 16, 1976), a biochemist at the Baylor College of Medicine, conducted an experiment which showed that THIQs were produced in the brains of rats when acetaldehyde combined chemically with dopamine. Davis and Walsh published their results in the February 13, 1970 issue of Science (see reference 1).
In 1983, Birgitta Sjöquist et al. of the Karolinska Institute in Sweden, published a paper reporting that autopsies of alcoholic and non-alcoholic subjects showed a non-significant trend for more THIQs to be present in the brains of alcoholics than non-alcoholics (see reference 2). Since the trend was not significant, it could have been the result of mere chance, and no conclusions should have been drawn from it. But instead, it resulted in one of the most bizarre theories in the history of addiction science.
A Theory Debunked
This was THIQ theory, which stated that alcohol turned into THIQs, which were essentially heroin, in the brains of alcoholics, but did not do so in the brains of social drinkers. THIQ theory was based on numerous false assumptions, the first of which was that “alcoholics” were as categorically different from “non-alcoholics” as Mendel’s green and yellow peas were from each other. The reality is that susceptibility to addiction to alcohol lies on a continuum, it is not a dichotomous variable. Another false assumption was that opiates like heroin are uniquely and instantaneously addictive, which they are not. Another problem is that many people who like alcohol dislike opiates, and vice versa. Finally, THIQ theory equated alcohol withdrawal with opiate withdrawal, despite the fact that the two have very little in common. THIQ theory was finally abandoned when it was shown that alcohol is just as likely to give rise to THIQs in the brains of non-alcoholics as it was in the brains of alcoholics.
Beyond a Theory – The Importance of THIQs
However, this does not mean that THIQs don’t play an important role in the actions of alcohol in the brain: they do. But before we go there, let us first take a look at the effects of alcohol on the brain’s opioid and dopamine systems.
As discussed above, in the most common neurotransmission scenario, receptors are found on the dendrite terminal (the postsynaptic terminal), and they receive neurotransmitters released from the axon terminal (the presynaptic terminal). However, this is not the case with the opioid receptors which control the release of dopamine into the nucleus accumbens. In this case, the opioid receptors are located on axon terminal (the presynaptic terminal) of GABA-releasing neurons. When agonists bind to these opioid receptors, they send a chemical message into the axon terminal which tells it to not release GABA into the synapse. In other words, these opioid receptors act to inhibit neurotransmission. Figure 1 shows the presynaptic GABA-releasing neuron with opioid receptors on its axon and the postsynaptic dopamine releasing neuron.
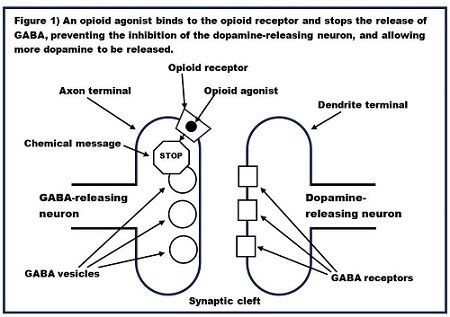
Note that, whereas in the rest of the brain alcohol is increasing the activity of the GABA neurons, the exact opposite is happening with these GABA neurons which control the release of dopamine into the nucleus accumbens.
What is the agonist which binds to the opioid receptor of the GABA-releasing neuron and where does it come from? Early on it was thought this agonist might be one of the brain’s naturally occurring (endogenous) opioids such as beta-endorphin or one of the enkephalins, since alcohol causes the release of beta endorphin from the hypothalamus and the pituitary.
However, more recent research shows that this agonist is almost certainly a THIQ known as salsolinol (1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline). A 2022 paper by Valentina Bassareo et al. shows that when salsolinol is prevented from forming in the brain, the release of dopamine into the nucleus accumbens fails to occur. Moreover, Andy Tseng et al. (2013) have shown that mice lacking beta-endorphin and mice lacking enkephalins still are subject to the reinforcing effects of alcohol which are a result of the release of dopamine into the nucleus accumbens. These experiments, taken together, strongly suggest that salsolinol is the agonist responsible for the dopamine release, rather than beta-endorphin or the enkephalins.
Like the other THIQs, salsolinol is formed when acetaldehyde combines chemically with dopamine. How does salsolinol reach the opioid receptors? All the neurons in the brain are constantly bathed in a soup called the interstitial fluid, which is filled with neurotransmitters and other chemicals. Salsolinol diffuses through this soup to reach the opioid receptors and trigger the release of dopamine. And that’s how a habit of drinking alcohol is formed.
Liked this article? You might also be interested in: The Genetics of Alcohol Withdrawal
References (unlinked):
- https://www.science.org/doi/abs/10.1126/science.167.3920.1005
- https://www.sciencedirect.com/science/article/abs/pii/0376871683900509
